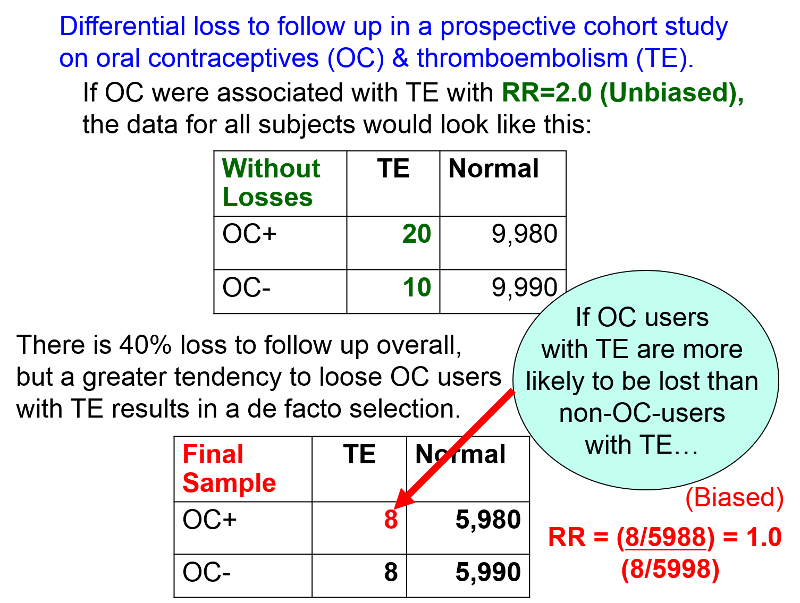
Cohort Study Bias . Inappropriate selection of participants into the cohort study can result in selection bias. Immortal time bias is increasingly common in cohort studies of drug effects. Cohort studies are at serious risk of confounding bias and so adjusting or accounting for confounding factors is a priority in these. The cohort study design is an excellent method to understand an outcome or the natural history of a disease or condition in an. We define bias as the systematic difference between the study results obtained from an nrsi and a pragmatic randomized trial (both with a. The characteristic feature of a cohort study is that the investigator identifies subjects at a point in time when they do not have the.
from sphweb.bumc.bu.edu
Immortal time bias is increasingly common in cohort studies of drug effects. The characteristic feature of a cohort study is that the investigator identifies subjects at a point in time when they do not have the. The cohort study design is an excellent method to understand an outcome or the natural history of a disease or condition in an. Inappropriate selection of participants into the cohort study can result in selection bias. Cohort studies are at serious risk of confounding bias and so adjusting or accounting for confounding factors is a priority in these. We define bias as the systematic difference between the study results obtained from an nrsi and a pragmatic randomized trial (both with a.
Cohort Studies
Cohort Study Bias Cohort studies are at serious risk of confounding bias and so adjusting or accounting for confounding factors is a priority in these. We define bias as the systematic difference between the study results obtained from an nrsi and a pragmatic randomized trial (both with a. The cohort study design is an excellent method to understand an outcome or the natural history of a disease or condition in an. The characteristic feature of a cohort study is that the investigator identifies subjects at a point in time when they do not have the. Cohort studies are at serious risk of confounding bias and so adjusting or accounting for confounding factors is a priority in these. Immortal time bias is increasingly common in cohort studies of drug effects. Inappropriate selection of participants into the cohort study can result in selection bias.
From www.slideserve.com
PPT Cohort Study PowerPoint Presentation, free download ID3161288 Cohort Study Bias We define bias as the systematic difference between the study results obtained from an nrsi and a pragmatic randomized trial (both with a. Cohort studies are at serious risk of confounding bias and so adjusting or accounting for confounding factors is a priority in these. Inappropriate selection of participants into the cohort study can result in selection bias. Immortal time. Cohort Study Bias.
From www.slideserve.com
PPT Lecture 8 Selection Bias, Matching, & Control Selection Cohort Study Bias The characteristic feature of a cohort study is that the investigator identifies subjects at a point in time when they do not have the. We define bias as the systematic difference between the study results obtained from an nrsi and a pragmatic randomized trial (both with a. Inappropriate selection of participants into the cohort study can result in selection bias.. Cohort Study Bias.
From www.slideshare.net
Bias and confounding in Cohort and case control study Cohort Study Bias Cohort studies are at serious risk of confounding bias and so adjusting or accounting for confounding factors is a priority in these. The cohort study design is an excellent method to understand an outcome or the natural history of a disease or condition in an. We define bias as the systematic difference between the study results obtained from an nrsi. Cohort Study Bias.
From www.researchgate.net
Differences between crosssectional, casecontrol, and cohort study Cohort Study Bias Cohort studies are at serious risk of confounding bias and so adjusting or accounting for confounding factors is a priority in these. The cohort study design is an excellent method to understand an outcome or the natural history of a disease or condition in an. The characteristic feature of a cohort study is that the investigator identifies subjects at a. Cohort Study Bias.
From en.wikipedia.org
Prospective cohort study Wikipedia Cohort Study Bias Cohort studies are at serious risk of confounding bias and so adjusting or accounting for confounding factors is a priority in these. The characteristic feature of a cohort study is that the investigator identifies subjects at a point in time when they do not have the. The cohort study design is an excellent method to understand an outcome or the. Cohort Study Bias.
From www.slideserve.com
PPT Cohort Studies PowerPoint Presentation, free download ID704357 Cohort Study Bias We define bias as the systematic difference between the study results obtained from an nrsi and a pragmatic randomized trial (both with a. Cohort studies are at serious risk of confounding bias and so adjusting or accounting for confounding factors is a priority in these. The characteristic feature of a cohort study is that the investigator identifies subjects at a. Cohort Study Bias.
From www.researchgate.net
(PDF) Cohort studies Sources of bias Cohort Study Bias Immortal time bias is increasingly common in cohort studies of drug effects. We define bias as the systematic difference between the study results obtained from an nrsi and a pragmatic randomized trial (both with a. The cohort study design is an excellent method to understand an outcome or the natural history of a disease or condition in an. The characteristic. Cohort Study Bias.
From www.slideshare.net
Bias and confounding in Cohort and case control study Cohort Study Bias The cohort study design is an excellent method to understand an outcome or the natural history of a disease or condition in an. Inappropriate selection of participants into the cohort study can result in selection bias. The characteristic feature of a cohort study is that the investigator identifies subjects at a point in time when they do not have the.. Cohort Study Bias.
From www.slideserve.com
PPT Epidemiology Cohort studies II March 2010 PowerPoint Cohort Study Bias Immortal time bias is increasingly common in cohort studies of drug effects. The characteristic feature of a cohort study is that the investigator identifies subjects at a point in time when they do not have the. The cohort study design is an excellent method to understand an outcome or the natural history of a disease or condition in an. Inappropriate. Cohort Study Bias.
From www.slideserve.com
PPT Cohort Study PowerPoint Presentation, free download ID3161288 Cohort Study Bias Immortal time bias is increasingly common in cohort studies of drug effects. Inappropriate selection of participants into the cohort study can result in selection bias. Cohort studies are at serious risk of confounding bias and so adjusting or accounting for confounding factors is a priority in these. The cohort study design is an excellent method to understand an outcome or. Cohort Study Bias.
From www.slideshare.net
Cohort and casecontrols studies Cohort Study Bias Immortal time bias is increasingly common in cohort studies of drug effects. The characteristic feature of a cohort study is that the investigator identifies subjects at a point in time when they do not have the. We define bias as the systematic difference between the study results obtained from an nrsi and a pragmatic randomized trial (both with a. Cohort. Cohort Study Bias.
From sphweb.bumc.bu.edu
Selection Bias in Cohort Studies Cohort Study Bias We define bias as the systematic difference between the study results obtained from an nrsi and a pragmatic randomized trial (both with a. The characteristic feature of a cohort study is that the investigator identifies subjects at a point in time when they do not have the. Cohort studies are at serious risk of confounding bias and so adjusting or. Cohort Study Bias.
From www.slideserve.com
PPT Cohort Study Designs PowerPoint Presentation, free download ID Cohort Study Bias Immortal time bias is increasingly common in cohort studies of drug effects. Inappropriate selection of participants into the cohort study can result in selection bias. We define bias as the systematic difference between the study results obtained from an nrsi and a pragmatic randomized trial (both with a. Cohort studies are at serious risk of confounding bias and so adjusting. Cohort Study Bias.
From sphweb.bumc.bu.edu
Selection Bias in Cohort Studies Cohort Study Bias Cohort studies are at serious risk of confounding bias and so adjusting or accounting for confounding factors is a priority in these. We define bias as the systematic difference between the study results obtained from an nrsi and a pragmatic randomized trial (both with a. Inappropriate selection of participants into the cohort study can result in selection bias. Immortal time. Cohort Study Bias.
From bmcmedresmethodol.biomedcentral.com
Evaluating selection bias in a populationbased cohort study with low Cohort Study Bias The cohort study design is an excellent method to understand an outcome or the natural history of a disease or condition in an. Inappropriate selection of participants into the cohort study can result in selection bias. We define bias as the systematic difference between the study results obtained from an nrsi and a pragmatic randomized trial (both with a. The. Cohort Study Bias.
From www.slideserve.com
PPT Bias PowerPoint Presentation ID1324959 Cohort Study Bias The cohort study design is an excellent method to understand an outcome or the natural history of a disease or condition in an. Inappropriate selection of participants into the cohort study can result in selection bias. The characteristic feature of a cohort study is that the investigator identifies subjects at a point in time when they do not have the.. Cohort Study Bias.
From www.slideserve.com
PPT Cohort Study Designs PowerPoint Presentation, free download ID Cohort Study Bias The cohort study design is an excellent method to understand an outcome or the natural history of a disease or condition in an. Inappropriate selection of participants into the cohort study can result in selection bias. Cohort studies are at serious risk of confounding bias and so adjusting or accounting for confounding factors is a priority in these. We define. Cohort Study Bias.
From www.slideshare.net
Bias and confounding in Cohort and case control study Cohort Study Bias We define bias as the systematic difference between the study results obtained from an nrsi and a pragmatic randomized trial (both with a. The characteristic feature of a cohort study is that the investigator identifies subjects at a point in time when they do not have the. Immortal time bias is increasingly common in cohort studies of drug effects. Inappropriate. Cohort Study Bias.